Independent career at UGA
2026
110. Valueva, A. D.; Chopra, E. M.; Klepov, V. V.* Unraveling Mechanism of Photoluminescence in Hybrid Indium Halide Materials
Adv. Opt. Mater. 2026 (accepted)
Rising Star 2025 special issue109. Valueva, A. D.; Chopra, E. M.; Long, H. A.; Novikov, S. A.; Klepov, V. V.* Photoluminescence Enhancement through Anion Sublattice Engineering in a Series of (dien)In(Cl1-xBrx)6 Hybrid Halides
Chem. Mater. 2026 DOI: 10.1021/acs.chemmater.5c02901
Honoring the Outstanding Contributions of Mercouri Kanatzidis to Chemistry of Materials special issue108. Patel, K. G.; Osakwe, A. R.; Fenn, G.; Crane, G. H.; Klepov, V. V.; Ritchie, B. W.; Locklin, J. J.* Partial Miscibility-Driven Nucleation of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) with Indigo Dye
ACS Appl. Polym. Mater. 2026 , 8 (3), 1648–1656. DOI: 10.1021/acsapm.5c03430

107. Long, H. A.; Smith, H. E.; Morrison, G.; Klepov, V. V.* CCompositional Tuning of Magnetic Properties in a Series of Transition Metal Site-Deficient UCoxBi2 and UNixBi2 Phases
Inorg. Chem. 2026 , 65 (2), 1611–1620. DOI: 10.1021/acs.inorgchem.5c05232
Quantum Materials from an Inorganic Chemistry Perspective special issue
106. Novikov S. A.; Gabilondo, E.; Valueva, A. D.; Locklin, J.; Klepov, V. V.* Noncentrosymmetric hybrid gallium halides built from non-polar inorganic units.
J. Mat. Chem. C 2026 DOI: 10.1039/D5TC03829D
Emerging Investigators special issue
105. Solovei, J.; Valueva, A. D.; Klepov, V. V.* Characterization of Hybrid Material (C2H5NH3)2[SbBr5]
J. Chem. Crystallogr. 2026 56 (1), 18. DOI: 10.1007/s10870-025-01076-z
Undergraduate first author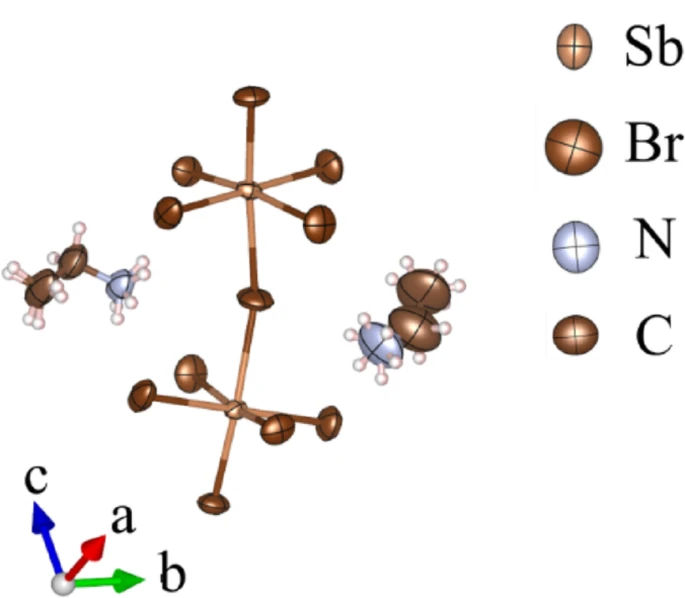
2025
104. Thippanna, V.; Sun, X.; Sobczak, M. T.; Ramanathan, A.; Theobald, T. G.; Doran, I.; Were, J.; Yang, L.; Jaraczewski, J.; Casey, J.; Klepov, V. V.; Liang, L.; Nolet, S.; Mada Kannan, A. N.; Xu, X.; Song, K. Turbine-to-Textile: Upcycling Wind Turbine Blade Waste into High-Performance PAN Composite Fibers.
ACS Appl. Polym. Mater. 2025, 7 (21), 14188–14200. DOI: 10.1021/acsapm.5c02466

103. Tabatadze, M.; Valueva, A. D.; Long, H. A.; Novikov S. A.; Zhao, Y.; Klepov V. V.* Cubic Cesium Lead Bromide Stabilized by Ethylammonium Incorporation
Inorg. Chem. 2025, 64 (32), 16433–16440. DOI: 10.1021/acs.inorgchem.5c02183

102. Valueva, A. D.; Novikov S. A.; Gabilondo, E.; Tisdale, H. B.; Maksimova, A. A.; Parker, M.; Reukov, V.; and Klepov V. V.* Hybrid Bismuth Halide with Rich Polymorphism and Second Harmonic Generation Response
ACS Mater. Lett. 2025, 7 (8), 2814–2821. DOI: 10.1021/acsmaterialslett.5c00784
Special issue "Emerging Investigators in Materials Science”.
101. Wood, C. C.; Patel, K. G.; Weber, V. L.; Osakwe, A. R.; Manovacia Moreno, N. P.; Broich, M. L.; Bledsoe, J. C.; Bramhall, J. A.; Klepov, V. V.; Bell, S.; Locklin, J. J.* Development of Impact Resistant Immediate Release Amorphous Solid Dispersion via Hot-Melt Extrusion and Injection Molding
Int. J. Pharm. 2025, 680, 125746. DOI: 10.1016/j.ijpharm.2025.125746

100. Novikov, S. A.; Casey, J.; Long, H. A.; Klepov, V. V.* Structural evolution and bonding features of electron deficient copper chalcogenides
CrystEngComm 2025, 27, 4787–4795. DOI: 10.1039/D5CE00479A

99. Long, H. A.; Waldis, O. G.; Klepov, V. V.* Synthesis of U0.92Mn3Si2C Using Organic Carbon Source
Z. Anorg. Allg. Chem. 2025, 651, e202500047. DOI: 10.1002/zaac.202500047
Invited contribution for Professor Gordon J. Miller 65th Birthday.
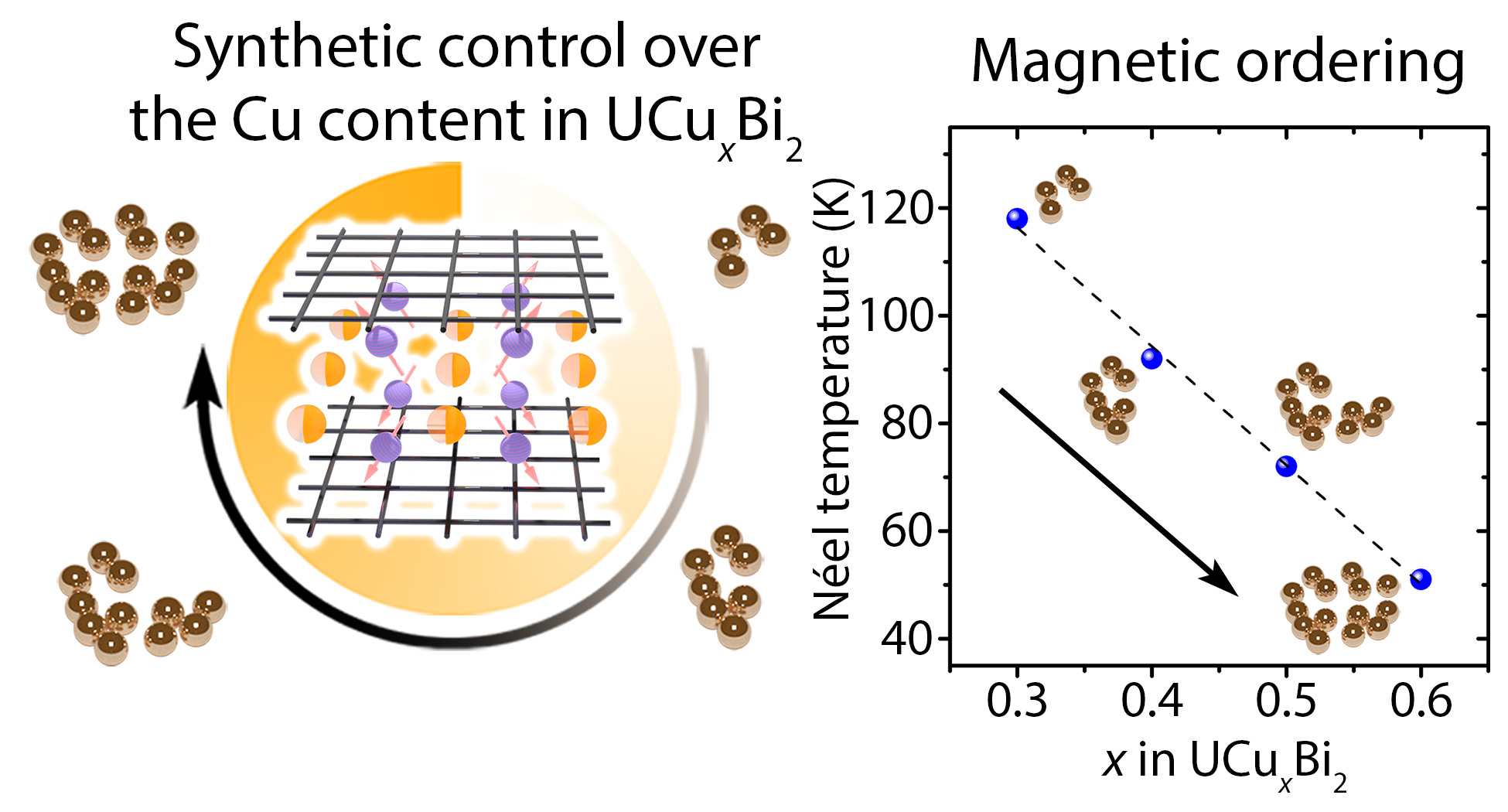
98. Long, H. A.; Duong, D.; Blawat, J.; Morrison, G.; Wu, Y.; Cao, H.; Pokhrel, N.; Parker, D.; Singleton, J.; Jin, R.; Klepov, V. V.* Magnetic Properties Tuning via Broad Range Site Deficiency in Square Net Material UCuxBi2
J. Am. Chem. Soc. 2025, 147 (18), 15157–15169. DOI: 10.1021/jacs.4c18438
97. Keerthisinghe, N.; Morrison, G.; Breton, L. S.; Smith, M. D.; Zhang, Q.; Kirkham, M. J.; Klepov, V. V.; zur Loye, H.-C.* From Magnetic Chains to Sheets: The Impact of Hydrofluoric Acid Quantities in Mild Hydrothermal Synthesis on Mixed-Metal Fluoride Formation
Chem. Mater. 2025, 37 (5), 2014–2025. DOI: 10.1021/acs.chemmater.4c03524

2024
96. Novikov, S. A.; Long, H. A.; Valueva, A. D.; Klepov, V. V.* Application of Voronoi Polyhedra for Analysis of Electronic Dimensionality in Emissive Halide Materials
J. Am. Chem. Soc. 2024, 146 (51), 35449–35461. DOI: 10.1021/jacs.4c14554

95. Novikov, S. A.; Valueva, A. D.; Klepov, V. V.* Bandgap Engineering and Photoluminescence Tuning in Halide Double Perovskites
Dalton Trans. 2024, 53 (30), 12442–12449. DOI: 10.1039/D4DT01420K

94. Mirmohammadsadeghi, S.; Juhas, D.; Parker, M.; Peranidze, K.; Van Horn, D. A.; Sharma, A.; Patel, D.; Sysoeva, T. A.; Klepov, V.; Reukov, V. The Highly Durable Antibacterial Gel-like Coatings for Textiles.
Gels 2024, 10 (6), 398. DOI: 10.3390/gels10060398

93. Tran, P. M; Wang, Y.; Dzikovski, B.; Lahm, M. E.; Xie, Y.; Wei, P.; Klepov, V. V.; Schaefer III, H. F.; Robinson, G. H. A Stable Aluminum Tris(dithiolene) Triradical
J. Am. Chem. Soc. 2024, 146 (23), 16340–16347. DOI: 10.1021/jacs.4c05631

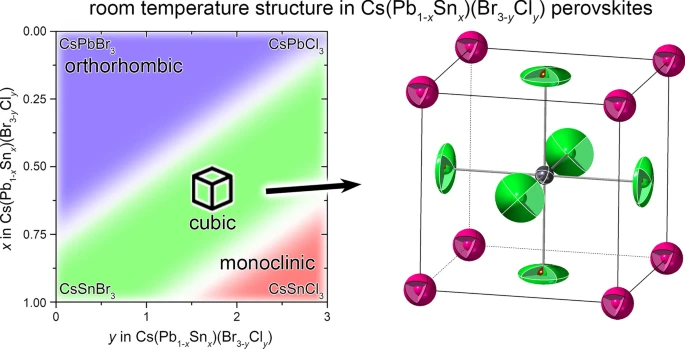
92. Valueva, A.D.; Novikov, S.A.; Bledsoe, J.; Cai, Y.; Maksimova, A.A.; Locklin, J.; Zhao, Y.; Klepov, V.* Cubic Halide Perovskites in the Cs(Pb1-xSnx)(Br3-yCly) Solid Solutions for Crack-Free Bridgman Grown Single Crystals
MRS Comm. 2024, 14, 942–948. DOI: 10.1557/s43579-024-00535-6
Early Career Materials Researcher issue
2023
91. Bledsoe, J.; Crane, G.; Drewke, J.; Casey, J.; Klepov, V.; Locklin, J. Balancing Melt Solubility and Morphology in Epitaxial Nucleation: The Case of Nicotinic Acid and Poly(hydroxybutyrate-co-hydroxyhexanoate)
ACS Appl. Polym. Mater. 2023, 5 (10), 8600–8607. DOI: 10.1021/acsapm.3c01692

90. Novikov, S. A.; Casey, J.; Long, H. A.; Bledsoe, J. C.; Locklin, J. J.; Klepov, V. V.* Electron deficiency in 2D chalcogenide NaCu4S3 with metallic properties.
Cryst. Growth Des. 2023, 23 (10), 7243–7251. DOI: 10.1021/acs.cgd.3c00649
Highlighted in Emerging Investigators in Crystal Growth & Design 2025
Prior to UGA
2024
89. Berseneva, A. A.; Klepov, V. V.; Kashem, H. B.; Maksimova, A. A.; Morrison, G.; Wright, J.; Schaeperkoetter, J.; Misture, S. T.; zur Loye, H.-C. A Rare Bird: U+5 in Uranium Chalcogenides.
Chem Mater. 2024, 36 (16), 7988–8001. DOI: 10.1021/acs.chemmater.4c01440

88. Balvanz, A.; Safdari, M.; Zacharias, M.; Kim, D.; Welton, C.; Oriel, E. H.; Kepenekian, M.; Katan, C.; Malliakas, C. D.; Even, J.; Klepov, V.; Manjunatha Reddy, G. N.; Schaller, R. D.; Chen, L. X.; Seshadri, R.; Kanatzidis, M. G. Structural Evolution and Photoluminescence Quenching across the FASnI3-xBrx (x = 0–3) Perovskites.
J. Am. Chem. Soc. 2024, 146 (23), 16128–16147. DOI: 10.1021/jacs.4c03669

2023
87. Liu, Z.; Peters, J.; Bayikadi, K.; Klepov, V.; Pan, L.; Pandey, I.; Kanatzidis, M. G.; Wessels, B. Defects of perovskite semiconductor CsPbBr3 investigated via photoluminescence and thermally stimulated current spectroscopies
J. Appl. Phys. 2023, 134, 245101. DOI: 10.1063/5.0177809

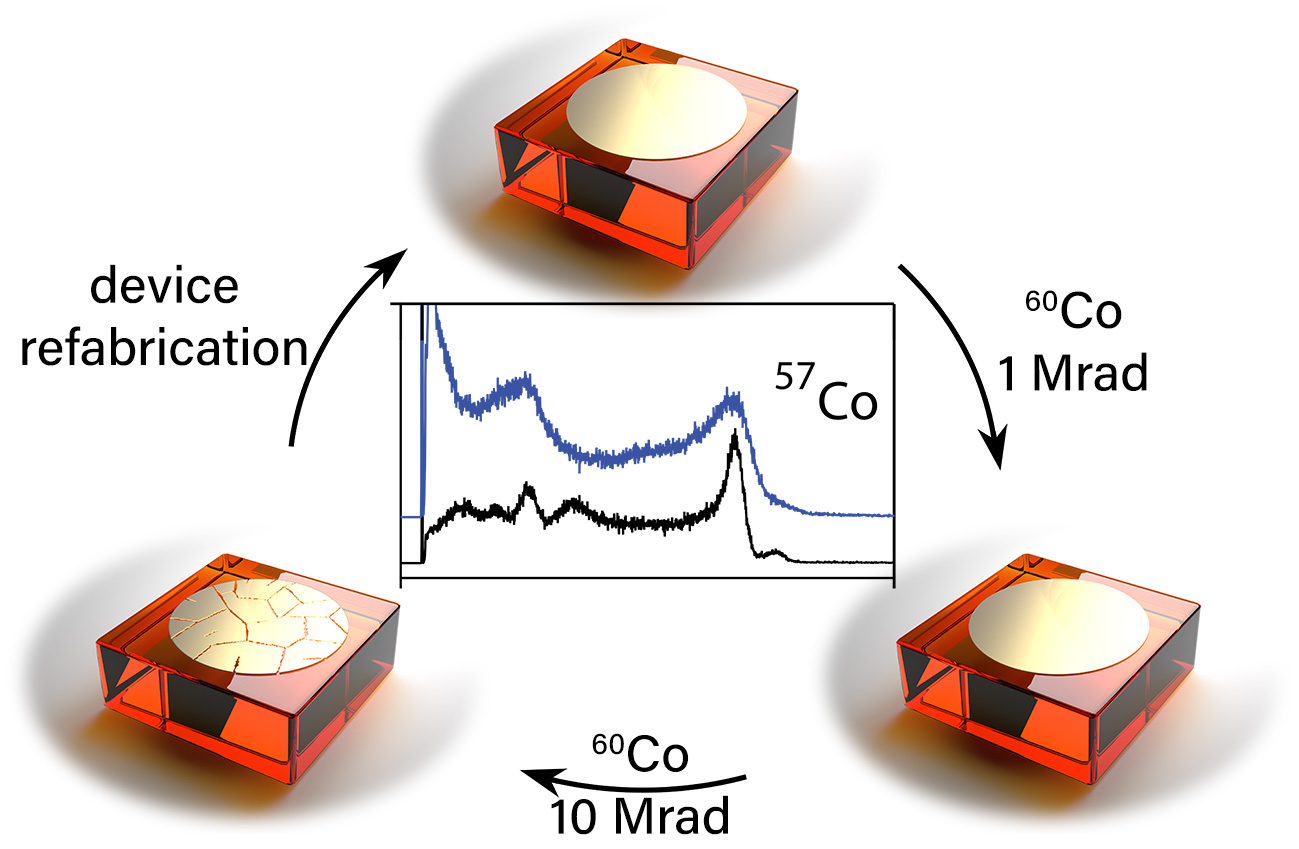
86. De Siena, M. C.#; Klepov, V. V.#; Stepanoff, S. P.; Bayikadi, K. S.; Pan, L.; Pandey, I. R.; Karki, S.; Chung, D. Y.; Wolfe, D. E.; Kanatzidis, M. G. Extreme γ‐Ray Radiation Tolerance of Spectrometer‐Grade CsPbBr3 Perovskite Detectors.
Adv. Mater. 2023, 2303244. DOI: 10.1002/adma.202303244
85. Vasileiadou, E. S.; Tajuddin, I. S.; De Siena, M. C.; Klepov, V. V.; Kepenekian, M.; Volonakis, G.; Even, J.; Wojtas, L.; Spanopoulos, I.; Zhou, X.; Iyer, A. K.; Fenton, J. L.; Dichtel, W. R.; Kanatzidis, M. G. Novel 3D Cubic Topology in Hybrid Lead Halides with a Symmetric Aromatic Triammonium Exhibiting Water Stability.
Chem. Mater. 2023, 35 (14), 5267–5280. DOI: 10.1021/acs.chemmater.3c00164

84. Pan, L.; Liu, Z.; Welton, C.; Klepov, V. V.; Peters, J. A.; De Siena, M. C.; Benadia, A.; Pandey, I.; Miceli, A.; Chung, D. Y.; Reddy, G. N. M.; Wessels, B. W.; Kanatzidis, M. G. Ultrahigh‐Flux X‐ray Detection by a Solution‐Grown Perovskite CsPbBr3 Single‐Crystal Semiconductor Detector.
Adv. Mater. 2023, 2211840. DOI: 10.1002/adma.202211840

83. Klepov, V. V.; De Siena, M. C.; Pandey, I. R.; Pan, L.; Bayikadi, K. S.; Butun, S.; Chung, D. Y.; Kanatzidis, M. G. Laser Scribing for Electrode Patterning of Perovskite Spectrometer-Grade CsPbBr3 Gamma-Ray Detectors.
ACS Appl. Mater. Interfaces 2023, 15 (13), 16895–16901. DOI: 10.1021/acsami.3c01212

82. Berseneva, A. A.; Klepov, V. V.; Tisdale, H. B.; zur Loye, H.-C. Flux-Assisted Polytypism in the [Na2Cl]GaQ2 Heterolayered Salt-Inclusion Chalcogenide Family.
CrystEngComm 2023, 25 (15), 2307–2312. DOI: 10.1039/D3CE00074E

81. Liu, Z.; Peters, J. A.; Pan, L.; Klepov, V.; De Siena, M.; Benadia, A.; Chung, D. Y.; Kanatzidis, M. G.; Wessels, B. W. Investigation of Defects in Melt and Solution Grown Perovskite CsPbBr3 Single Crystals.
Appl. Phys. Lett. 2023, 122 (13), 131902. DOI: 10.1063/5.0142802

80. Pan, L.; Pandey, I. R.; Miceli, A.; Klepov, V. V.; Chung, D. Y.; Kanatzidis, M. G. Perovskite CsPbBr3 Single‐Crystal Detector Operating at 1010 Photons s-1 mm-2 for Ultra‐High Flux X‐ray Detection.
Adv. Opt. Mater. 2023, 2202946. DOI: 10.1002/adom.202202946

2022
79. Fu, P.; Quintero, M. A.; Welton, C.; Li, X.; Cucco, B.; De Siena, M. C.; Even, J.; Volonakis, G.; Kepenekian, M.; Liu, R.; Laing, C. C.; Klepov, V.; Liu, Y.; Dravid, V. P.; Manjunatha Reddy, G. N.; Li, C.; Kanatzidis, M. G. Short Aromatic Diammonium Ions Modulate Distortions in 2D Lead Bromide Perovskites for Tunable White-Light Emission.
Chem. Mater. 2022, 34 (21), 9685–9698. DOI: 10.1021/acs.chemmater.2c02471

78. Pan, L.; He, Y.; Klepov, V. V.; De Siena, M. C.; Kanatzidis, M. G. Perovskite CsPbBr3 Single Crystal Detector for High Flux X-Ray Photon Counting.
IEEE Trans. Med. Imaging 2022, 41 (11), 3053–3061. DOI: 10.1109/TMI.2022.3176801
77. Vasileiadou, E. S.; Jiang, X.; Kepenekian, M.; Even, J.; De Siena, M. C.; Klepov, V. V.; Friedrich, D.; Spanopoulos, I.; Tu, Q.; Tajuddin, I. S.; Weiss, E. A.; Kanatzidis, M. G.* Thick-Layer Lead Iodide Perovskites with Bifunctional Organic Spacers Allylammonium and Iodopropylammonium Exhibiting Trap-State Emission.
J. Am. Chem. Soc. 2022. 144 (14) 6390-6409. DOI: 10.1021/jacs.2c00571

76. He, Y.; Hadar, I.; De Siena, M. C.; Klepov, V. V.; Pan, L.; Chung, D. Y.; Kanatzidis, M. G.* Sensitivity and Detection Limit of Spectroscopic‐Grade Perovskite CsPbBr3 Crystal for Hard X‐Ray Detection.
Adv. Funct. Mater. 2022, 32 (24), 2112925. DOI: 10.1002/adfm.202112925

75. Berseneva, A. A.; Klepov, V. V.; Pal, K.; Seeley, K.; Koury, D.; Schaeperkoetter, J.; Wright, J. T.; Misture, S. T.; Kanatzidis, M. G.; Wolverton, C.; Gelis, A. V.; zur Loye, H.-C.* Transuranium Sulfide via the Boron Chalcogen Mixture Method and Reversible Water Uptake in the NaCuTS3 Family.
J. Am. Chem. Soc. 2022, 144 (30), 13773–13786. DOI: 10.1021/jacs.2c04783

74. Novikov, S. A.; Lu, Y.; Zhang, W.; Halasyamani, P. S.; Hariyani, S.; Brgoch, J.; Klepov, V. V.; zur Loye, H.-C.; Mozharivskyj, Y.* Phosphorescence in Mn4+-Doped R+/R2+ Germanates (R+ = Na+ or K+, R2+ = Sr2+).
Inorg. Chem. 2022, 61 (24), 9364–9374. DOI: 10.1021/acs.inorgchem.2c01364

73. Carone, D.; Klepov, V. V.; Misture, S. T.; Schaeperkoetter, J. C.; Jacobsohn, L. G.; Aziziha, M.; Schorne-Pinto, J.; Thomson, S. A. J.; Hines, A. T.; Besmann, T. M.; zur Loye, H.-C.* Luminescence and Scintillation in the Niobium Doped Oxyfluoride Rb4Ge5O9F6:Nb.
Inorganics 2022, 10 (6), 83. DOI: 10.3390/inorganics10060083

72. Kutahyali Aslani, C.; Klepov, V. V.; zur Loye, H.-C.* Hydrothermal Synthesis of new Mixed-Oxoanion Materials: Rare Earth Iodate Sulfates Sm(IO3)(SO4) and Ln2(IO3)3(SO4)OH∙3H2O (Ln = Sm, Eu, Dy).
Solid State Sci. 2022. 129, 106918. DOI: 10.1016/j.solidstatesciences.2022.106918

71. Keerthisinghe, N.; Chsritian, M.S.; Berseneva, A. A.; Morrison, G.; Klepov, V. V.; Smith M.D.; zur Loye, H.-C.* Investigation of Metastable Low Dimensional Halometallates.
Molecules. 2022. 27 (1), 280. DOI: 10.3390/molecules27010280

2021
70. Torma, A.; Li, W.; Zhang, H.; Tu, Q.; Klepov, V. V.; Brennan, M. C.; McCleese, C. L.; Krzyaniak, M. D.; Wasielewski, M. R.; Katan, C.; Even, J.; Holt, M. V.; Grusenmeyer, T. A.; Jiang, J.; Pachter, R.; Kanatzidis, M. G.; Blancon, J.-C.*; Mohite, A. D.* Interstitial nature of Mn2+ doping in 2D perovskites.
ACS Nano. 2021. 15 (12), 20550-20561. DOI: 10.1021/acsnano.1c09142

69. Peters, J. A.; Liu, Z.; Bulgin, O.; He, Y.; Klepov, V. V.; De Siena, M. C.; Kanatzidis, M. G.; Wessels, B. W.* Excitons in CsPbBr3 Halide Perovskite.
J. Phys. Chem. Lett. 2021, 12 (38), 9301–9307. DOI: 10.1021/acs.jpclett.1c02397

68. Klepov, V. V.; Pace, K. A.; Berseneva, A. A.; Felder, J. B.; Calder, S.; Morrison, G.; Zhang, Q.; Kirkham, M. J.; Parker, D. S.; zur Loye, H.-C.* Chloride reduction of Mn3+ in the mild hydrothermal synthesis of a charge ordered defect pyrochlore, CsMn2+Mn3+F6, a canted antiferromagnet with a hard ferromagnetic component.
J. Am. Chem. Soc. 2021, 143 (30), 11554-11567. DOI: 10.1021/jacs.1c04245

67. Keerthisinghe, N.; Berseneva, A. A.; Klepov, V. V.; Morrison, G.; zur Loye, H.-C.* A Geometrically Frustrated Family of MIIMIIIF5(H2O)2 Mixed–Metal Fluorides with Complex Magnetic Interactions.
Inorg. Chem. 2021, 60 (18), 14318–14329. DOI: 10.1021/acs.inorgchem.1c01889

66. Christian, M. S.*; Pace, K. A.; Klepov, V. V.; Morrison, G.; zur Loye, H.-C.*; Besmann, T. M.* A Density-Functional Theory Structural Database for Discovery of Novel Actinide Waste Forms.
Cryst. Growth Des. 2021, 21 (9), 5100–5107. DOI: 10.1021/acs.cgd.1c00494

65. Kutahyali Aslani, C.; Klepov, V. V.; Aslani, M.; zur Loye, H.-C.* Hydrothermal Synthesis of new Iodates Ln2(IO3)3(IO4) (Ln=La, Nd, Pr) Containing the Tetraoxoiodate(V) Anion: Creation of Luminescence Properties by Doping with Eu, Dy, Tb.
Cryst. Growth Des. 2021, 21 (8), 4707-4712. DOI: 10.1021/acs.cgd.1c00545

64. Pace, K. A.; Klepov, V. V.; Smith, M. D.; Williams, T.; Morrison, G.; Lauterbach, J. A.; Misture, S. T.; zur Loye, H.-C.* Hydrothermal Synthesis and Structural Investigation of a Crystalline Uranyl Borosilicate.
Inorganics 2021, 9 (4), 25. DOI: 10.3390/inorganics9040025
63. Morrison, G.; Klepov, V. V.; zur Loye, H.-C.* Pentanary Cesium Titanyl/Titanate Silicate Oxyfluorides: Syntheses and Structures.
Solid State Sci. 2021, 118, 106664. DOI: 10.1016/j.solidstatesciences.2021.106664

62. Kutahyali Aslani, C.; Breton, L. B.; Klepov, V. V.; zur Loye, H.-C.* A Series of Rb4Ln2(P2S6)(PS4)2 (Ln = La, Ce, Pr, Nd, Sm, Gd) Rare Earth Thiophosphates with Two Distinct Thiophosphate Units [PVS4]3- and [PIV2S6]4-.
Dalton Trans. 2021, 50, 1683-1689. DOI: 10.1039/D0DT03718D

61.* Pace, K. A.§; Klepov, V. V. §*; Berseneva, A. A.; zur Loye, H.-C.* Covalency in Actinide Compounds.
Chem. Eur. J. 2021, 27 (19), 5835–5841. DOI: 10.1002/chem.202004632

60. Klepov, V. V.; Kocevski, V.; Besmann, T. M.; zur Loye, H.-C.* Dimensional Reduction upon Calcium Incorporation in Cs0.3(Ca0.3Ln0.7)PS4 and Cs0.5(Ca0.5Ln0.5)PS4.
CrystEngComm 2021, 23, 831-840. DOI: 10.1039/D0CE01524E
59. Kutahyali Aslani, C.; Klepov, V. V.; zur Loye, H.-C.* Flux Crystal Growth of a New BaTa2O6 Polymorph, and of the Novel Tantalum Oxyfluoride Salt Inclusion Phase [Ba3F]Ta4O12F: Flux Dependent Phase Formation.
J. Solid State Chem. 2021, 294, 121833. DOI: 10.1016/j.jssc.2020.121833

2020
58.* Breton, L. B.§; Klepov, V. V.§*; zur Loye, H.-C.* Facile Oxide to Chalcogenide Conversion for Actinides using the Boron-Chalcogen Mixture Method. (§equal contribution)
J. Am. Chem. Soc. 2020, 142 (33), 14365–14373. DOI: 10.1021/jacs.0c06483

57. Carone, D.; Usman, M.; Klepov, V. V.; Smith, M. D.; Kocevski, V.; Besmann, T. M.; zur Loye, H.-C.* New Germanate and Mixed Cobalt Germanate Salt Inclusion Materials: [(Rb6F)(Rb4F)][Ge14O32] and [(Rb6F)(Rb3.1Co0.9F0.96)][Co3.8Ge10.2O30F2].
CrystEngComm 2020, 22 (46), 8072–8080. DOI: 10.1039/D0CE01099E

56. Ejegbavwo, O. A.; Berseneva, A. A.; Martin, C. R.; Leith, G. A.; Pandey, S.; Brandt, A. J.; Park, K. C.; Mathur, A.; Farzandh, S.; Klepov, V. V.; Heiser, B. J.; Chandrashekhar, M.; Karakalos, S. G.; Smith, M. D.; Phillpot, S. R.; Garashchuk, S.; Chen, D. A.*; Shustova, N. B.* Heterometallic Multinuclear Nodes Directing MOF Electronic Behavior.
Chem. Sci. 2020, 11 (28), 7379–7389. DOI: 10.1039/D0SC03053H
55. Pace, K. A.; Klepov, V. V.; Deason, T. K.; Smith, M. D.; Ayer, G. B.; DiPrete, D. P.; Amoroso, J. W.; zur Loye, H.-C.* Expansion of the Na3MIII(Ln/An)6F30 Series: Incorporation of Plutonium into a Highly Robust and Stable Framework.
Chem. Eur. J. 2020, 26, 12941–12944. DOI: 10.1002/chem.202002774

54. Ayer, G. B.; Klepov, V. V.; Smith, M. D.; Hu, M.; Yang, Z.; Martin, C. R.; Morrison, G.; zur Loye, H.-C.* BaWO2F4: A Mixed Anion X-ray Scintillator with Excellent Photoluminescence Quantum Efficiency.
Dalton Trans. 2020, 49, 10734–10739. DOI: 10.1039/D0DT02184A

53. Keerthisinghe, N.; Klepov, V. V.; Zhang, E.; Smith, M. D.; Egodawatte, S.; Foulger, S. H.; zur Loye, H.-C.* Hydrothermal Synthesis and Properties of MMF5(H2O)7 (M = Co2+ and Ni2+, M = Mn3+, Ga3+, and In3+).
Solid State Sci. 2020, 108, 106374. DOI: 10.1016/j.solidstatesciences.2020.106374

52. Pace, K. A.; Klepov, V. V.; Christian, M. S.; Morrison, G.; Deason, T. K.; Kutahyali Aslani, C.; Besmann, T. M.; Diprete, D. P.; Amoroso, J. W.; zur Loye, H.-C.* Targeting Complex Plutonium Oxides by Combining Crystal Chemical Reasoning with Density-Functional Theory Calculations: The Quaternary Plutonium Oxide Cs2PuSi6O15.
Chem. Commun. 2020, 56 (66), 9501–9504. DOI: 10.1039/D0CC02674C
51. Klepov, V. V.; Berseneva, A. A.; Pace, K. A.; Kocevski, V.; Sun, M.; Qiu, P.; Wang, H.; Chen, F.; Besmann, T. M.; zur Loye, H.-C.* NaGaS2: An Elusive Layered Compound with Dynamic Water Absorption and Wide-Ranging Ion-Exchange Properties.
Angew. Chem. Int. Ed. 2020, 59 (27), 10836–10841. DOI: 10.1002/anie.202001203

50. Juillerat, C. A.; Klepov, V. V.; Smith, M. D.; zur Loye, H.-C.* Targeted Crystal Growth of Uranium Gallates Via the Systematic Exploration of the UF4-GaPO4-ACl (A = Cs, Rb) Phase Space.
CrystEngComm 2020, 22, 3020–3032. DOI: 10.1039/D0CE00343C

49.* Klepov, V. V.*; Juillerat, C. A.; Pace, K. A.; Morrison, G.; zur Loye, H.-C.* “Soft” Alkali Bromide and Iodide Fluxes for Crystal Growth.
Front. Chem. 2020, 8, 518. DOI: 10.3389/fchem.2020.00518
48. Ayer, G. B.; Klepov, V. V.; Pace, K. A.; zur Loye, H.-C.* Quaternary Cerium(IV) Containing Fluorides Exhibiting Ce3F16 Sheets and Ce6F30 Frameworks.
Dalton Trans. 2020, 49, 5998–5905. DOI: 10.1039/D0DT00616E

47. Pace, K. A.; Koch, R. J.; Smith, M. D.; Morrison, G.; Klepov, V. V.; Besmann, T. M.; Misture, S. T.; zur Loye, H.-C.* Crystal Growth of Alkali Uranyl Borates from Molten Salt Fluxes: Characterization and Ion Exchange Behavior of A2(UO2)B2O5 (A = Cs, Rb, K).
Inorg. Chem. 2020, 59 (9), 6449–6459. DOI: 10.1021/acs.inorgchem.0c00536

46. Juillerat, C. A.; Kocevski, V.; Klepov, V. V.; Amoroso, J. W.; Besmann, T. M.; zur Loye H.-C.* Structure and Stability of Alkali Gallates Structurally Reminiscent of Hollandite.
J. Am. Ceram. Soc. 2020, 103 (11), 6531–6542. DOI: 10.1111/jace.17327
45. Klepov, V. V.; Pace, K. A.; Breton, L. S.; Kocevski, V.; Besmann, T. M.; zur Loye, H.-C.* Nearly Identical but not Isotypic – Influence of the Lanthanide Contraction on Cs2NaLn(PS4)2 (Ln = La–Nd, Sm, Gd–Ho).
Inorg. Chem. 2020, 59 (3), 1905–1916. DOI: 10.1021/acs.inorgchem.9b03200

44. Uhanov, A. S.; Klepov, V. V.; Vologzhanina, A. V.; Zubavichus, Y. V.; Savchenkov, A. V.*; Pushkin, D. V.; Serezhkina, L. B.; Serezhkin, V. N. New Itaconate-Containing Uranyl Complex Unit and Coordination Modes of Itaconate Ions.
Comptes Rendus. Chimie 2020, 23 (2), 117–126. DOI: DOI: 10.5802/crchim.8
43. Hao, Y.; Alekseev, E. V.*; Klepov, V. V.; Yu, N.* Two Structural Variations in Complex Sodium Thorium Arsenates.
Eur. J. Inorg. Chem. 2020, 2020 (33), 3187–3193. DOI: 10.1002/ejic.202000492

2019
42.* Chekhomova, O. A.; Klepov, V. V.*; Pushkin, D. V.; Alekseev, E. V.; Vologzhanina, A. V.; Serezhkina, L. B.; Serezhkin, V. N. Structural Features of Uranyl Acrylate Complexes with s-, p-, and d-Monovalent Metals.
Z. Krist. Cryst. Mater. 2019, 234 (4), 247–256. DOI: 10.1515/zkri-2018-2089
41. Carone, D.; Klepov, V. V.; Smith, M. D.; zur Loye, H.-C.* Flux Crystal Growth of Lanthanide Tungsten Oxychlorides, La8.64W6O30.45Cl, Ce8.64W5.74O30Cl, and Ln8.33W6O30Cl (Ln = Pr, Nd): Structural Stability in the Presence of Extreme Cation and Anion Disorder.
Inorg. Chem. 2019, 58 (24), 16831–16837. DOI: 10.1021/acs.inorgchem.9b03015

40. Ayer, G. B.; Klepov, V. V.; Smith, M.; zur Loye, H.-C.* Mild Hydrothermal Synthesis of the Complex Hafnium Containing Fluorides Cs2[M(H2O)6][Hf2F12] (M= Ni, Co, and Zn), CuHfF6(H2O)4 and Cs2Hf3Mn3F20 Containing HfF7 and HfF6 Coordination Polyhedra.
Inorg. Chem. 2019, 58 (19), 13049–13057. DOI: 10.1021/acs.inorgchem.9b01958

39. Usman, M.; Smith, M.; Klepov, V. V.; zur Loye, H.-C.* One-Dimensional Quaternary and Penternary Alkali Rare Earth Thiophosphates Obtained via Alkali Halide Flux Crystal Growth.
Cryst. Growth Des. 2019, 19 (10), 5648–5657. DOI: 10.1021/acs.cgd.9b00637

38. Usman, M.; Smith, M. D.; Morrison G.; Klepov, V. V.; Zhang, W.; Halasyamani, P. S.; zur Loye, H.-C.* Molten Alkali Halide Flux Growth of an Extensive Family of Noncentrosymmetric Rare Earth Sulfides: Structure and Magnetic and Optical (SHG) Properties.
Inorg. Chem. 2019, 58 (13), 8541–8550. DOI: 10.1021/acs.inorgchem.9b00849

37. Klepov, V. V.; Smith, M. D.; zur Loye, H.-C.* Targeted Synthesis of Uranium(IV) Thiosilicates.
Inorg. Chem. 2019, 58 (13), 8275–8278. DOI: 10.1021/acs.inorgchem.9b01307

36. Klepov, V. V.; Breton, L. S.; Pace, K. A.; Kocevski, V.; Besmann, T. M.; zur Loye, H.-C.* Size-Driven Stability of Lanthanide Thiophosphates Grown from an Iodide Flux.
Inorg. Chem. 2019, 58 (9), 6565–6573. DOI: 10.1021/acs.inorgchem.9b00806

35. Klepov, V. V.; Pace, K. A.; Calder, S.; Felder, J. B.; zur Loye, H.-C.* 3d-Metal Induced Magnetic Ordering on U(IV) Atoms as a Route toward U(IV) Magnetic Materials.
J. Am. Chem. Soc. 2019, 141 (9), 3838–3842. DOI: 10.1021/jacs.9b00345

34. Klepov, V. V.; Morrison, G.; zur Loye, H.-C.* NanMTh6F30: A Large Family of Quaternary Thorium Fluorides.
Cryst. Growth Des. 2019, 19 (2), 1347–1355. DOI: 10.1021/acs.cgd.8b01742

33. Klepov, V. V.§; Juillerat, C. A. §; Alekseev, E. V.; zur Loye, H.-C.* Overstepping Löwenstein’s Rule - A Route to Unique Aluminophosphate Frameworks with Three-Dimensional Salt-Inclusion and Ion-Exchange Properties.
Inorg. Chem. 2019, 58 (1), 724–736. (§equal authorship) DOI: 10.1021/acs.inorgchem.8b02906

32. Juillerat, C. A. §; Klepov, V. V. §; Morrison, G.; Pace, K. A.; zur Loye, H.-C.* Flux Crystal Growth: A Versatile Technique to Reveal the Crystal Chemistry of Complex Uranium Oxides.
Dalton Trans. 2019, 48 (10), 3162–3181. (§equal authorship) DOI: 10.1039/C8DT04675A
31. Usman, M.; Morrison, G.; Klepov, V. V.; Smith, M. D.; zur Loye, H.-C.* Flux Crystal Growth, Structure, Magnetic and Optical Properties of a Family of Alkali Uranium(IV) Phosphates.
J. Solid State Chem. 2019, 270, 19–26. DOI: 10.1016/j.jssc.2018.10.033

30. Serezhkina, L. B.*; Grigoriev, M. S.; Klepov, V. V.; Shimin, N. A.; Serezhkin, V. N. Synthesis and Structure of Strontium and Barium Uranyl Methacrylates.
Crystallogr. Rep. 2019, 64 (2), 270–276. DOI: 10.1134/S1063774519020251
2018
29. Serezhkin, V. N.*; Savchenkov, A. V.; Klepov, V. V.; Stefanovich, S. Y.; Pushkin, D. V.; Serezhkina, L. B. Relationship between the Structure and Nonlinear Optical Properties of R[UO2L3] and R3[UO2L3]4 Crystals (L—Carboxylate Ion).
Russ. J. Inorg. Chem. 2018, 63 (5), 647–654. DOI: 10.1134/S0036023618050145
28. Klepov, V. V.; Serezhkina, L. B.*; Grigor’ev, M. S.; Ignatenko, E. O.; Serezhkin, V. N. Uranyl Methacrylate Complexes with Carbamide and Methylcarbamide: Synthesis and Structure.
Russ. J. Inorg. Chem. 2018, 63 (8), 1019–1025. DOI: 10.1134/S0036023618080119
27. Pace, K. A.; Klepov, V. V.; Morrison, G.; zur Loye, H.-C.* Moderate Supercritical Synthesis as a Facile Route to Mixed-Valent Uranium(IV,V) and (V,VI) Silicates.
Chem. Comm. 2018, 54 (98), 13794–13797. DOI: 10.1039/C8CC07789D
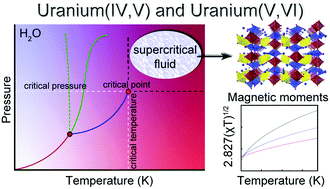
26. Klepov, V. V.; zur Loye, H.-C.* Complex Topologies from Simple Building Blocks: Uranium(IV) Thiophosphates.
Inorg. Chem. 2018, 57 (17), 11175–11183. DOI: 10.1021/acs.inorgchem.8b01733

25. Klepov, V. V.; Felder, J. B.; zur Loye, H.-C.* Synthetic Strategies for the Synthesis of Ternary Uranium(IV) and Thorium(IV) Fluorides.
Inorg. Chem. 2018, 57 (9), 5597–5606. DOI: 10.1021/acs.inorgchem.8b00570

24. Li, H.; Kegler, P.; Klepov, V. V.; Klinkenberg, M.; Bosbach, D.; Alekseev, E. V.* Comparison of Uranium(VI) and Thorium(IV) Silicates Synthesized via Mixed Fluxes Techniques.
Inorg. Chem. 2018, 57 (11), 6734–6745. DOI: 10.1021/acs.inorgchem.8b01072

23. Hao, Y.; Klepov, V. V.; Kegler, P.; Modolo, G.; Bosbach, D.; Albrecht-Schmitt, T. E.; Wang, S.; Alekseev, E. V.* Synthesis and Study of the First Zeolitic Uranium Borate.
Cryst. Growth Des. 2018, 18 (1), 498–505. DOI: 10.1021/acs.cgd.7b01487

Prior 2018
22. Yu, N.; Klepov, V. V.; Schlenz, H.; Bosbach, D.; Kowalski, P. M.; Li, Y.; Alekseev, E. V.* Cation-Dependent Structural Evolution in A2Th(TVO4)2 (A = Li, Na, K, Rb, Cs; T = P and As) Series.
Cryst. Growth Des. 2017, 17 (3), 1339–1346. DOI: 10.1021/acs.cgd.6b01741

21.* Klepov, V. V.*; Serezhkina, L. B.; Grigoriev, M. S.; Shimin, N. A.; Stefanovich, S. Y.; Serezhkin, V. N. Morphotropy in Alkaline Uranyl Methacrylate Complexes.
Polyhedron 2017, 133, 40–47. DOI: 10.1016/j.poly.2017.04.041

20.* Klepov, V. V.*; Vologzhanina, A. V.; Alekseev, E. V.; Pushkin, D. V.; Serezhkina, L. B.; Sergeeva, O. A.; Knyazev, A. V.; Serezhkin, V. N. Structural Diversity of Uranyl Acrylates.
CrystEngComm 2016, 18 (10), 1723–1731. DOI: 10.1039/C5CE01957E
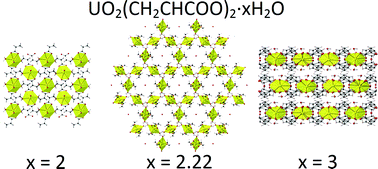
19.* Klepov, V. V.*; Serezhkina, L. B.; Serezhkin, V. N.; Alekseev, E. V.* Synthesis and Crystal Structure Analysis of Uranyl Triple Acetates.
J. Solid State Chem. 2016, 244, 100–107. DOI: 10.1016/j.jssc.2016.09.019

18.* Klepov, V. V.*; Serezhkina, L. B.; Pushkin, D. V.; Alekseev, E. V.; Grigor’ev, M. S.; Sergeeva, O. A.; Shimin, N. A.; Serezhkin, V. N. Uranyl Complexes with (Meth)Acrylate Anions.
Eur. J. Inorg. Chem. 2016, 2016 (1), 118–125. DOI: 10.1002/ejic.201501035

17. Xiao, B.; Kegler, P.; Gesing, T. M.; Robben, L.; Blanca-Romero, A.; Kowalski, P. M.; Li, Y.; Klepov, V.; Bosbach, D.; Alekseev, E. V.* Giant Volume Change and Topological Gaps in Temperature- and Pressure-Induced Phase Transitions: Experimental and Computational Study of ThMo2O8.
Chem. Eur. J. 2016, 22 (3), 946–958. DOI: DOI: 10.1002/chem.201503839

16. Hao, Y.; Klepov, V. V.; Murphy, G. L.; Modolo, G.; Bosbach, D.; Albrecht-Schmitt, T. E.; Kennedy, B. J.; Wang, S.; Alekseev, E. V.* Influence of Synthetic Conditions on Chemistry and Structural Properties of Alkaline Earth Uranyl Borates. Cryst.
Growth Des. 2016, 16 (10), 5923–5931. DOI: 10.1021/acs.cgd.6b00978

15*. Yu, N.; Klepov, V. V.*; Neumeier, S.; Depmeier, W.; Bosbach, D.; Suleimanov, E. V.; Alekseev, E. V.* Further Insight into Uranium and Thorium Metaphosphate Chemistry and the Effect of Nd3+ Incorporation into Uranium(IV) Metaphosphate.
Eur. J. Inorg. Chem. 2015, 2015 (9), 1562–1568. DOI: 10.1002/ejic.201403098
14. Yu, N.; Kegler, P.; Klepov, V. V.; Dellen, J.; Schlenz, H.; Langer, E. M.; Bosbach, D.; Alekseev, E. V.* Influence of Extreme Conditions on the Formation and Structures of Caesium Uranium(VI) Arsenates.
Dalton Trans. 2015, 44 (47), 20735–20744. DOI: 10.1039/C5DT03842A
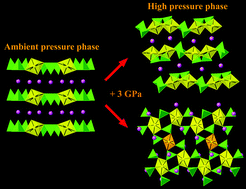
13. Serezhkina, L. B.*; Grigor’ev, M. S.; Shimin, N. A.; Klepov, V. V.; Serezhkin, V. N. First Uranyl Methacrylate Complexes: Synthesis and Structure.
Russ. J. Inorg. Chem. 2015, 60 (6), 672–683. DOI: 10.1134/S0036023615060121
12. Savchenkov, A. V.*; Klepov, V. V.; Vologzhanina, A. V.; Serezhkina, L. B.; Pushkin, D. V.; Serezhkin, V. N. Trinuclear {Sr[UO2L3]2(H2O)4} and Pentanuclear {Sr[UO2L3]4}2- Uranyl Monocarboxylate Complexes (L - Acetate or n-Butyrate Ion).
CrystEngComm 2015, 17 (4), 740–746. DOI: 10.1039/C4CE02103G
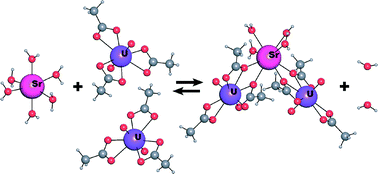
11. Yu, N.; Klepov, V. V.; Villa, E. M.; Bosbach, D.; Suleimanov, E. V.; Depmeier, W.; Albrecht-Schmitt, T. E.*; Alekseev, E. V.* Topologically Identical, but Geometrically Isomeric Layers in Hydrous α-, β-Rb[UO2(AsO3OH)(AsO2(OH)2)]·H2O and Anhydrous Rb[UO2(AsO3OH)(AsO2(OH)2)].
J. Solid State Chem. 2014, 215, 152–159. DOI: 10.1016/j.jssc.2014.03.017

10. Yu, N.; Klepov, V. V.; Modolo, G.; Bosbach, D.; Suleimanov, E. V.; Gesing, T. M.; Robben, L.; Alekseev, E. V.* Morphotropy and Temperature-Driven Polymorphism in A2Th(AsO4)2 (A = Li, Na, K, Rb, Cs) Series.
Inorg. Chem. 2014, 53 (20), 11231–11241. DOI: 10.1021/ic5018246

9. Yu, N.; Klepov, V. V.; Kegler, P.; Bosbach, D.; Albrecht-Schmitt, T. E.*; Alekseev, E. V.* Th(AsIII4AsV4O18): A Mixed-Valent Oxoarsenic(III)/Arsenic(V) Actinide Compound Obtained under Extreme Conditions.
Inorg. Chem. 2014, 53 (16), 8194–8196. DOI: 10.1021/ic5013704

8.* Klepov, V. V.*; Serezhkina, L. B.; Vologzhanina, A. V.; Pushkin, D. V.; Sergeeva, O. A.; Stefanovich, S. Y.; Serezhkin, V. N. Tris(Acrylato)Uranylates as a Scaffold for NLO Materials.
Inorg. Chem. Comm. 2014, 46, 5–8. DOI: 10.1016/j.inoche.2014.04.024

7. Klepov, V. V.; Vologzhanina, A. V.; Serezhkina, L. B.*; Serezhkin, V. N. Synthesis, Structure, and Properties of [Be(H2O)4][UO2(CH3COO)3]2.
Radiochem. 2013, 55 (1), 36–40. DOI: 10.1134/S1066362213010074
6.* Klepov, V. V.*; Peresypkina, E. V.; Serezhkina, L. B.; Karasev, M. O.; Virovets, A. V.; Serezhkin, V. N. Crystal Structure of [M(H2O)6][UO2(CH3COO)3]2 (M = Mg2+, Co2+ and Zn2+).
Polyhedron 2013, 61, 137–142. DOI: 10.1016/j.poly.2013.05.048

5. Klepov, V. V.; Peresypkina, E. V.; Serezhkina, L. B.; Virovets, A. V.; Serezhkin, V. N.* Synthesis and Structure of [Cr3O(CH3COO)6(H2O)3][UO2(CH3COO)3]·3H2O.
Russ. J. Inorg. Chem. 2012, 57 (10), 1341–1347. DOI: 10.1134/S0036023612050129
4. Serezhkina, L. B.*; Vologzhanina, A. V.; Klepov, V. V.; Serezhkin, V. N. Synthesis and X-Ray Diffraction Study of (Cs0.5Ba0.25)[UO2(CH3COO)3] and Ba0.5[UO2(CH3COO)3].
Crystallog. Rep. 2011, 56 (2), 265–269. DOI: 10.1134/S1063774511010214
3.Serezhkina, L. B.*; Vologzhanina, A. V.; Klepov, V. V.; Serezhkin, V. N. Crystal Structure of PbUO2(CH3COO)4(H2O)3.
Crystallogr. Rep. 2011, 56 (1), 132–135. DOI: 10.1134/S1063774510061100
2. Serezhkina, L. B.*; Vologzhanina, A. V.; Klepov, V. V.; Serezhkin, V. N. Crystal Structure of R[UO2(CH3COO)3] (R = NH4+, K+, or Cs+).
Crystallogr. Rep. 2010, 55 (5), 773–779. DOI: 10.1134/S1063774510050093
1. Serezhkina, L. B.*; Peresypkina, E. V.; Virovets, A. V.; Klepov, V. V. Synthesis and Structure of (Rb0.50Ba0.25)[UO2(CH3COO)3].
Crystallogr. Rep. 2010, 55 (2), 221–223. DOI: 10.1134/S1063774510020094